Environmental Measurements Lab--Titrations
A titration is a test carried out by adding a measured amount of a standardized reagent to a sample until some endpoint is reached. The endpoint is usually a color change or some other obvious transition. There is a defined relationship, such as a stoichiometric relationship, between the reagent and the substance of interest (the analyte) that allows the analyst to calculate the amount of the analyte in the sample. See Chapter 9 of your lab manual.
The reagent, called the titrant, is put into a burette. The analyst notes the starting volume and then adds the reagent slowly, stopping when the endpoint is reached. The initial volume is subtracted from the final volume to give the net volume added. Then the analyst does a C1V1=C2V2 calculation, with C1 = the concentration of the titrant, V1 = the volume of titrant added, V2 = the volume of sample to which the titrant was added and C2 = the unknown concentration of the sample.
You will do three types of titrations on water samples that you and your classmates bring in. You will do hardness by EDTA titration, as described in section 21.2 of your lab manual; total alkalinity using mixed indicator as described in section 20.14; and chloride as described below.
Chloride Measurements by the Argentometric Method
(Standard Methods 19th Ed. Method 4500-Cl-B)
In a neutral or slightly alkaline solution, potassium chromate gives a red end point in the silver nitrate titration of chloride after all the chloride is precipitated as silver chloride.
If silver ion (Ag+) is added to water which contains chloride ion (Cl-), the two combine to form silver chloride, which is very insoluble:
Ag+ + Cl- ® AgCl(ppt)
The "ppt" in parentheses after the reaction is used to indicate that the silver chloride precipitates (turns into a solid). Silver and chromate ion (CrO4-2) also combine to form silver chromate:
Ag+ + CrO4-2 ® Ag2CrO4(ppt)
This compound has a higher solubility product; as long as there is chloride present, any silver chromate that forms will disappear as the silver gets taken out of solution by the silver chloride. Once the chloride is gone, the silver chromate persists. This compound is red, so it is visible in a beaker of water.
To measure chloride in a water sample, we add some potassium chromate solution, which is yellow. To the yellow sample, we slowly add silver nitrate solution (a soluble form of nitrate). A red color may appear briefly as silver chromate forms and redissolves. The silver chloride which precipitates may not be noticeable because it is white. At some point, all the chloride will have been precipitated. We can tell that this point has been reached because the red color will stay. The color change is called the endpoint, because we stop adding silver nitrate once it has been reached. A test in which we slowly add one solution to another and then stop at some endpoint, is called a titration. The solution added, in this case silver nitrate, is called the titrant.
In order to determine the amount of chloride in the sample, we have to measure the amount of silver nitrate we add before the endpoint is reached. To do this we use a burette, which is a long glass tube marked with numbers (usually milliliters) and fitted with a valve or stopcock at the bottom. To use a burette, we write down the number that corresponds to the starting level of solution. After we have added as much solution as we need (ie, after we have reached the endpoint), we note the final level. We find the amount used by subtracting the starting number from the final number. Difference is the number of milliliters of solution used.
For the number of milliliters of titrant used to be meaningful, we need to know the amount of silver in each milliliter. We get this number by titrating a chloride solution of known chloride concentration. We know the concentration of the chloride standard because we make it up by weighing a precise amount of sodium chloride and adding a measured volume of water. To get the molarity of the silver nitrate solution, we use this formula:
Molarity AgNO3 = M NaCl * mL NaCl/mL AgNO3
Note: This is C2 = C1 * V1/V2
To do these tests, we will need to make up a number of solutions. The solutions and other ingredients used to carry out tests or other lab procedures are called reagents.
Reagents:
Potassium chromate indicator solution: Dissolve 5 g K2CrO4 in about 5 mL distilled water (dH2O). Add a few drops of silver nitrate until a definite red precipitate forms. Let stand 12 hours, filter and dilute to 100 mL with dH2O.
Standard sodium chloride (0.0141 M): Dissolve 824.0 mg NaCl (dried at 140°C) in dH2O and dilute to 1000 mL. One milliliter of this solution contains how many milligrams of chloride?
Standard silver nitrate titrant (0.0141 M): Dissolve 2.395 g AgNO3 in dH2O and dilute to 1000 mL. Standardize against 0.0141 M NaCl. One milliliter of this solution will react with how many milligrams of chloride? (Click for answer.)
Procedure:
Measure 25 mL of sample with a graduated cylinder. If doing a standard, take 1.0 mL or other precise volume of standard sodium chloride solution made up to 25 mL with distilled water. Sample pH must be in the range 7 to 10. Add 5 drops of chromate indicator solution. Titrate with silver nitrate solution until a pinkish or reddish tinge persists in the yellow solution.
Titrate a couple of blanks, standards, and/or samples until you can consistently recognize the endpoint. Blanks would be just distilled water and typically require only one or two drops of titrant.
Calculation:
To get the concentration of chloride in a sample, use the following formula:
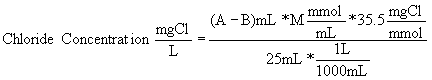
Where:
A = mL titrant used for sample
B = mL titrant used for blank (this might be 0 mL)
M = molarity of silver nitrate
Note: This is C2 = V1/V2 * C1 with concentrations converted from molar to mg/L. C1 and V1 refer to the titrant; C2 and V2 refer to the sample.
If the silver nitrate comes out at exactly 0.0141 M, then the formula reduces to:
mg Cl/L = (A-B)mL * (20.0 mgCl-/L)/mL
For example, if A were 1.65 mL and B were 0.05 mL, then:
Choride content = (1.65 - 0.05)mL * (20.0 mgCl-/L)/mL
= 32 mgCl/L
Lab Exercise
You have two weeks to complete this lab assignment. Do the following:
- Ask me any questions you have about what you are supposed to do for this assignment.
- Make any necessary reagents. Some may be made for you already. There will be a list of what needs to be made. We can divide up the work (in other words, not every group needs to make every reagent).
- Watch the instructor do one of each titration. Ask any questions you have about the procedures.
- Do a few practice titrations to familiarize yourself with the endpoint and other aspects of the procedure. Careful, the endpoints for chloride and for hardness are fairly subtle. When you repeat a titration, you should be able to get the same volume of titrant used plus or minus a drop or two (approximately 0.1 mL).
- When you are comfortable with a titration (ie, alkalinity, chloride, or hardness), standardize the titrant (ie, sulfuric acid, silver nitrate, or EDTA). Some of the solutions may already be standardized for you. You may be able to convince another group to give you some of their standardized reagent (but you still need to do number 5 below).
- Standardize the sulfuric acid against solid sodium carbonate as described on pages 68-69 of your lab manual. You will make and standardize a 0.1 N H2SO4 solution, which you will then dilute exactly 1 up to 5 (eg, 20 mL up to 100 mL) to get 0.02 N sulfuric acid.
- Standardize the silver nitrate solution against the standard 0.0141 M sodium chloride solution. Record all information in your observations notebook. Do three titrations and average the results of all three. Mark the silver nitrate bottle with the obtained concentration, your initials and the date.
- Standardize the EDTA solution against CaCO3 standard solution as described on page 241 of your lab manual. (Note: the line just below equation 21.7 should read "where 0.02 = prepared normality of CaCO3….")
- Do one of each titration on a QC standard (eg, WP32-2). There may be a separate QC standard for each titration. Calculate the concentrations of alkalinity, chloride, and hardness. Check your results against the true values and acceptance limits listed for the standards.
- Measure the alkalinity, chloride concentration and hardness on a water sample. Perform replicate measurements for chloride so that you can calculate a relative percent difference (RPD--see your textbook for more information). Post your RPD on the board because you will need the values for everyone in the class to complete your lab report. Each person should do this so that we end up with a number for each person in the class.
- Measure the chloride concentration of TRCTC-QC1. Post your result on the board. Each person should do this.
- You have two weeks to complete your lab report as described on the attached page. Ask any questions you have about what you did or about the report.
Required in Lab Report #3 (Due 7 Mar 01)
Title Page; Include
- Title
- Author’s name
- Lab partners’ names
- Date
Results
- a worksheet showing your silver nitrate standardization
- this should show the observations and calculations you made to standardize the silver nitrate solution. This should be neat and clear enough so that someone could decide whether or not to believe the concentration marked on the bottle.
- see attached samples
- a "report to your client"; this should be in letter or memo format
- date, your name and address, client’s name and address (use mine)
- info on samples: where from, collected when and by whom
- identify all samples for which data is reported
- values for chloride, hardness, and alkalinity
- if you do dupliclate analyses, report only the final average values
- present this clearly, accurately, and attractively
- this report should be signed by its author
- include your "approval number" and date issued
Control Chart for Accuracy
- use the results for the QC standard you titrated
- plot the data for everyone else in the class
- add your point as the last one
- see attached sample and page 36 in your lab manual
Control Chart for Precision
- use the RPD values from the repeated analyses
- see page 36 in your lab manual
Notebook Pages and Calculations
- you will have a lot of info here; be sure it is neat and complete
Appendix:
Sample Precision Control Chart with Data (This is a Microsoft Excel file. Internet Explorer users click on the link then choose File, Save As… to save the file on your computer. You can then edit it as you please.)(Netscape users should view this important information first.)
Others (View with Netscape/View with Internet Explorer):
Sample Notebook Pages for Titrant Standardization and Titration
Sample Letter Report (note: the information in this is not necessarily accurate)
Excerpt from Standard Methods (19th Ed.) (3 pages)
Anthony Benoit abenoit@trcc.commnet.edu