ENV 2101 Principles of Environmental Engineering
1. History
a. what is environmental engineering?
-environmental engineering is the branch of applied science that builds and operates things to protect people and the environment
-as a separate field it is relatively new, the term came into use during the 1960s
-the discipline was formed primarily from civil engineering and public health, informed by the science of ecology and to some extent by the philosophy of ethics)
-chemical engineering has its place in environmental engineering (especially here at TRCC) and mechanical, electrical, etc, all get involved at some point
-we are going to be concerned mostly with the operation (and to some extent the design) of systems used to improve the quality of some matrix for use or disposal
-water treatment
-wastewater treatment
-air scrubbers
-solid waste treatment and destruction
-alternative production processes
-"pollution prevention" is the new wave
-more product specific than point of discharge treatment systems
b. history
-in preshistory, humans lived as other animals do, by hunting and gathering their food
-10s or 100s of thousands of years ago, the stone age began when people started using stone tools and weapons and wood fueled fires for cooking and perhaps heating
-environmental problems began at once
-there is archaeological evidence that overhunting obliterating species in some areas, which would then be left by bands of humans
-there is also evidence of fires having been used inside caves
-about 10 thousand years ago, people began raising food
-this is the beginning of civilization
-environmental problems multiplied
-water supply had to be secured
-there are documents as old as 4000 years recommending that water be boiled before drinking
-body wastes, garbage, and other solid wastes had to be disposed of
-wood smoke degraded air quality
-engineering got its start around this time as an adjunct to defense of towns
-with towns came permanent structures to be defended
-with agriculture came specialization
-engineering was considered one of the arts of war up until the 19th century
-in classical times, the Romans made strides in what we would now call civil engineering (the term civil engineer comes to us from 18th century England)
-nine major aqueducts supplied water to the city; some as big as 50 feet across or 50 miles long
-the Industrial Revolution saw a rapid increase in urban population densities and attendent environmental problems
-the 18th and 19th centuries were one of increasing number and sophistication of water supplies
-quantity was the immediate concern, though filtration of water began in 1804 and chlorination was introduced in 1902 after the role of water in disease epidemics became known in the mid-1800s
-by 1920 the spread of waterborne illness had be greatly decreased
-the first water disposal problem to be examined by civil engineers was the routing of storm waters
-as early as 1700, the city of Boston had a storm drain system
-although it was often illegal to discharge wastes to the drainage system, as people had more water supplied to them it became more common to dispose of wastewater in the storm drains
-such combined sewers persist to this day in places such as Norwich and New York City
-these place a great strain on treatment plants and receiving waters
-city households that did not discharge to the storm drains typically had cesspools as late as the 1880s
-the high cost of building sewer systems inhibited their construction even though the subterranean systems had to be pumped every year or so (with the waste usually dumped on the ground outside of town)
-in the 1880s, sanitary sewers started to be built in the US
-Memphis TN had one of the first though it failed because the pipes were too small and were difficult to clean
-the years 1890 to 1900 saw a great number of municipal sanitary sewers built
-the storm drains and most of the early sewers emptied directly into the receiving water without treatment
-this was shall we say, unpleasant to those who live along the shore
-there is a story that the master of Trinity College responded to Queen Victoria's inquiry about floating pieces of paper in the River Cam by telling her that they were notices that swimming is prohibited
-even when wood was the main fuel, cities had a problem with poor air quality but the switch to coal made matters even worse
-copper and gold smelting as early as 4000 BC led to pockets of industrial air pollution
-in 61 BC, the Roman philosopher Seneca wrote that he felt better once he breathed the cleaner air outside the city
-in England in the late 13th and early 14th century, emissions from wood and soft coal combustion led to government regulation
-in 1661, air pollution in London provoked a government study that concluded that half of all deaths in the city were attributable to air pollution
-the invention of the steam engine in 1784 worsened the problem of air pollution in industrial settings
-engineering response to the problems of air pollution were not as magnificent as to those of water supply or water pollution
-smoke abatement ordinances were passed in Chicago and Cincinati in 1881; these mostly served to limit the types of fuel and hours of operation that were permitted in furnaces etc
-air quality continued to deteriorate
-in London in December 1952, a temperature inversion blocked a cloud of smog near the ground; combustion actually increased because the cloud blocked the sun and lowered surface temperatures; 4000 people died in London from breathing polluted air
-since that time London fogs have decreased in frequency due to the replacement of coal with natural gas as a heating fuel
-coal is still the predominant heating fuel in Beijing, China where locals suffer from what is called the "Beijing Cough"
-in this century automobiles have become a major source of air pollution
-combustion engineers are tackling this problem as we speak
c. ecology
-the word ecology, meaning the logical study of the interelationships between organisms and their surroundings, was invented in the mid-1800s
-the study of ecology blossomed in the 1960s
-basic to ecology is the ecosystem
-an ecosystem is a system: a set of interrelated parts having properties not found in the components
-an ecosystem includes the living participants (usually one or more populations-though a monoculture, with only one population, is extremely unstable) and the nonliving components or abiotic factors
-the population levels are dependent on the effects of each population on the others and on the effects of the abiotic factors on each
-another basic concept is limits or limiting factors
-biomass increases in an ecosystem until it is checked by the limiting factor
-this can be physical, chemical, etc; biological factors can limit the size of a population but usually do not limit biomass production
-similar to limiting factor is carrying capacity: the ability of an ecosystem to support a population or a level of biomass
-our environmental engineering skills will likely play a role in determining what the earth's carrying capacity for humans will be and what the quality or our lives will be within that capacity
2. Review of Units
-see appendix, page 639 in Masters
-engineers use both the metric system (or SI) and the English system (or USCS)
-basic units:
|
quantity |
SI |
USCS |
notes |
|
mass |
kg |
slug |
commonly we equate mass and weight by saying that 1 lb = 454 g |
|
length |
m |
ft |
km or miles are common in large scale measurement |
|
time |
s |
s |
min, hr, days etc |
|
force |
N |
lb |
|
|
temperature |
°C or K |
°F. or °R |
|
-other quantities are combinations of these:
|
quantity |
SI |
USCS |
notes |
|
volume |
m3 |
ft3 |
|
|
area |
m2 |
ft2 |
|
|
velocity |
m/s |
mi/hr |
|
|
flow rate |
m3/s |
ft3/s |
|
|
energy |
N· m or J |
Btu, ft-lb |
|
|
power |
N· m/s or W |
Btu/hr, hp |
|
|
density |
kg/m3 |
slug/ft3 |
|
-the metric systems is supposed to be easy because of a set of prefixes that are used to denote order of magnitude (somewhat like scientific notation)
|
10-15 |
femto |
f |
|
10-12 |
pico |
p |
|
10-9 |
nano |
n |
|
10-6 |
micro |
m or u |
|
10-3 |
milli |
m |
|
10-2 |
centi |
c |
|
10-1 |
deci |
d |
|
10 |
deca or deka |
da |
|
102 |
hecto or cento |
h or C |
|
103 |
kilo |
k |
|
106 |
mega |
M |
|
109 |
giga |
G |
|
1012 |
tera |
T |
|
1015 |
peta |
P |
|
1018 |
exa |
E |
-concentrations are expressed in a number of ways, primarily as mass per volume
-in chemical calculations we often have to convert to moles in order to equate quantities of input and output or to predict rates of reaction etc
-the conversion to moles is accomplished by multiplying by one mole for every N grams of material, where N is the gram-molecular weight of the substance
-to get gram-molecular weight for a chemical, add the atomic weights of each element in the chemical formula multiplied by the subscript if any
-thus for water it's:
H2O 2 * 1 plus 16 = 18 g/mol
-a pound of water is how many moles? (we are really asking the mass of water that weighs one pound is how many moles)
1 lb * 454 g/lb * 1 mol/18g = 25.2 mol
-if we completely burn a gallon of gasoline, how many lbs of CO2 do we produce? Assume that the formula for gasoline is C7H16 and that a gallon of gasoline has a mass of 2600 g. Assume the following reaction for combustion:
C7H16 + 11O2 ® 7CO2 + 8H2O
![]()
-Try this:
1000 kg of N are discharged into a lake; if nitrogen is the limiting nutrient and the empirical formula for algal biomass is C6H10O2N, what is the amount of algal biomass the lake can support? (Assume the one mole of nitrogen can produce one "mole" of algae.)
![]()
3. Mass balance
-an important concept that we will use is that of a mass balance
-the main idea is this: everything has to come from somewhere and go somewhere
-based on the conservation of mass
-to come up with a mass balance we need to pick a substance and define a boundary
-the simplest mass balance can be easily pictured and easily written algebraically:
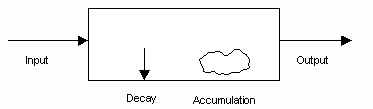
INPUT RATE = OUTPUT RATE + DECAY RATE + ACCUMULATION RATE
-not all of these terms will apply to all models
-a conservative substance does not decay within our system
-this can be further simplified by assuming steady-state or equilibrium conditions (no change in accumulation)
-these assume that accumulation rate is zero
-for a steady-state conservative system:
INPUT RATE = OUTPUT RATE
-how about a pollutant being carried by a stream into a lake?
QinCin = QoutCout
-are we making any other assumptions here?
-suppose that a stream flowing at 10.0 m3/s has a tributary coming in at 5.0 m3/s; the sodium concentration in the main stream is 20.0 mg/L but the feeder is contaminated with road runoff so its sodium concentration is 40.0 mg/L; assuming complete mixing, what is the sodium concentration downstream of the mixing point? (sodium is usually considered to be conservative in natural waters)
QsCs + QtCt = QoutCout
we also assume that
Qs + Qt = Qout
therefore:
Cout = (QsCs + QtCt)/Qout = (QsCs + QtCt)/(Qs + Qt)
= 26.7 mg/L
-for nonconservative pollutants, the decay can be chemical, biological or even radioactive
-the decay process is often modeled as a first order reaction; ie, the rate of decay (in mass per time) is assumed to be proportional to the amount of substance presence; in terms of concentration:
D
C/D t = -KC-if you know calculus, you can easily show that this situation leads to exponential decay:
C = C0e-Kt
ANTHONY G BENOIT abenoit@trcc.commnet.edu
(860) 885-2386
Revised 8/30/01